
Chemistry
Chemistry is the study of matter, its properties, how and why substances combine or separate to form other substances, and how substances interact with energy. Many people think of chemists as being white-coated scientists mixing strange liquids in a laboratory, but the truth is we are all chemists. Understanding basic chemistry concepts is important for almost every profession. Chemistry is part of everything in our lives, from growing and cooking food to cleaning our homes and bodies to launching a space shuttle. Chemistry is one of the physical sciences that help us to describe and explain our world.
Matter and Measurement
Click the button on the right to view the PowerPoint presentations, notes, and other resources for the Matter and Measurement Unit.
Unit 1 Learning Targets:
-
I can apply the scientific method to analyze a chemical reaction.
-
I can engineer a balance.
-
I can classify pure substances and mixtures.
-
I can classify matter using physical and chemical changes.
-
I can use conversion factors to solve metric conversion problems.
-
I can answer multiplication and division problems with correct significant figures.
-
I can measure the physical property of density to identify an unknown metal.
Atoms and the Periodic Table
Click the button on the right to view the PowerPoint presentations, notes, and other resources for the Atoms and the Periodic Table Unit.
Unit 2 Learning Targets:
-
I can describe the subatomic structure of an atom.
-
I can use the periodic table to determine the number of each subatomic particle for any isotope.
-
I can use the periodic table to predict the electron configuration and Bohr Model for any element.
-
I can explain trends in atomic radius within groups and periods on the periodic table.
-
I can convert between the number of atoms in an element and the mass of that element using the mole, Avogadro's number, and the atomic mass of that element.
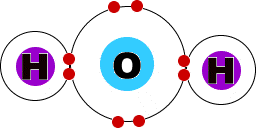
Covalent Compounds
Click the button on the right to view the PowerPoint presentations, notes, and other resources for the Covalent Compounds Unit.
Unit 3 Learning Targets:
-
I can name binary covalent compounds.
-
I can use Lewis Dot Structures to determine the structure of molecules.
-
I can predict the 3D structure of molecules using VSEPR theory.
-
I can determine the intermolecular attractive forces acting on polar and non-polar molecules.
-
I can predict the relative boiling point of elements, polar, molecules, and non-polar molecules.
-
I can calculate the molar mass for a compound.
-
I can convert between mass and moles using the molar mass of a molecule.
-
I can explain the action of a drinking bird toy using intermolecular attractive forces and the kinetic theory.

Ionic Compounds
Click the button on the right to view the PowerPoint presentations, notes, and other resources for the Ionic Compounds Unit.
Unit 4 Learning Targets:
-
I can use the periodic table to predict the ion that s and p block elements will form.
-
I can predict how the atomic radius changes for cations and anions.
-
I can describe the properties of ionic compounds.
-
I can write the name and chemical formula for ionic compounds.
-
I can predict the Lewis Dot structure and 3D geometry of polyatomic ions.
-
I can explain the patterns in groups and periods for ionization energy and electron affinity.
-
I can convert between mass and moles using the molar mass of an ionic compound.
Unit 5 Learning Targets:
-
I can identify the signs of a chemical change.
-
I can use the law of conservation of mass to balance chemical equations.
-
I can identify types of chemical reactions.
-
I can predict the products of single and double displacement reactions.
-
I can use mole ratios to convert between the moles of any two substances in a chemical reaction.
-
I can use mole ratios and molar mass to convert between the masses of any two substances in a chemical reaction.
-
I can determine if a chemical reaction is endothermic or exothermic.
-
I can use enthalpy, mole rations and molar mass to convert between heat, moles, and mass for any substance in a chemical reaction.
Chemical Reactions
Click the button on the right to view the PowerPoint presentations, notes, and other resources for the Chemical Reactions Unit.
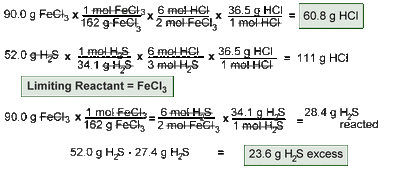
Stoichiometry
Click the button on the right to view the PowerPoint presentations, notes, and other resources for the Stoichiometry Unit.
Unit 6 Learning Targets:



